Sickle Cell Anaemia (SCA) is a disorder caused by a variant of a gene that affects haemoglobin, the oxygen-carrying molecule in red blood cells. If a person inherits one mutated gene, they carry the sickle cell trait but in the vast majority of cases will not develop SCA. But if a person inherits the mutated gene from both parents, then they will develop SCA.

The mutation causes haemoglobin to polymerise (stick together) which then deforms the red blood cell, causing it to take irregular shapes including a sickle shape. Consequently, these malformed blood cells can’t flow through the blood vessels properly which restricts blood supply to organs. This results in a vast array of problems, primarily hemolytic anemia (low blood caused by destruction of blood cells) and ischemic damage to tissues and organs resulting in frequent periods of severe pain and even organ failure. Acute chest syndrome is a typical example of organ failure in sickle cell disease and one of the leading causes of hospitalisation and death among patients (Piel et al 2017:1562-1565)

70% of babies born with SCA are born in Africa, most notably in Nigeria and the Democratic Republic of Congo. Although infant mortality (death before 5) among children with SCA has fallen in richer countries, it is still extremely high in Africa. A 2010 estimate put it at between 50-90% though obtaining accurate statistics on sickle cell in Africa is difficult. Screening new-borns for SCA is a vital component to being able to treat it as effectively as possible and this is normal practice in places like the UK, the US. To date no African country has implemented mass screening for the trait though some have conducted trial screenings (Kato et al 2018:2-3, 10).
The high prevalence of SCA in Africa is primarily explained by its connection with malaria. The so-called malaria hypothesis was first postulated by western scientists in the early twentieth century. The hypothesis begins by noting that malaria has been around for thousands of years and until recently has resulted in almost-certain death in infancy. These factors mean that it would have played a role in natural selection. Because malaria affects blood cells, any changes the structure of blood cells could also hinder the spread of malaria. Thus, it was hypothesised that genotypes associated with changes to the shape of blood cells should be more common in areas with high malaria prevalence (Luzzatto, 2012).
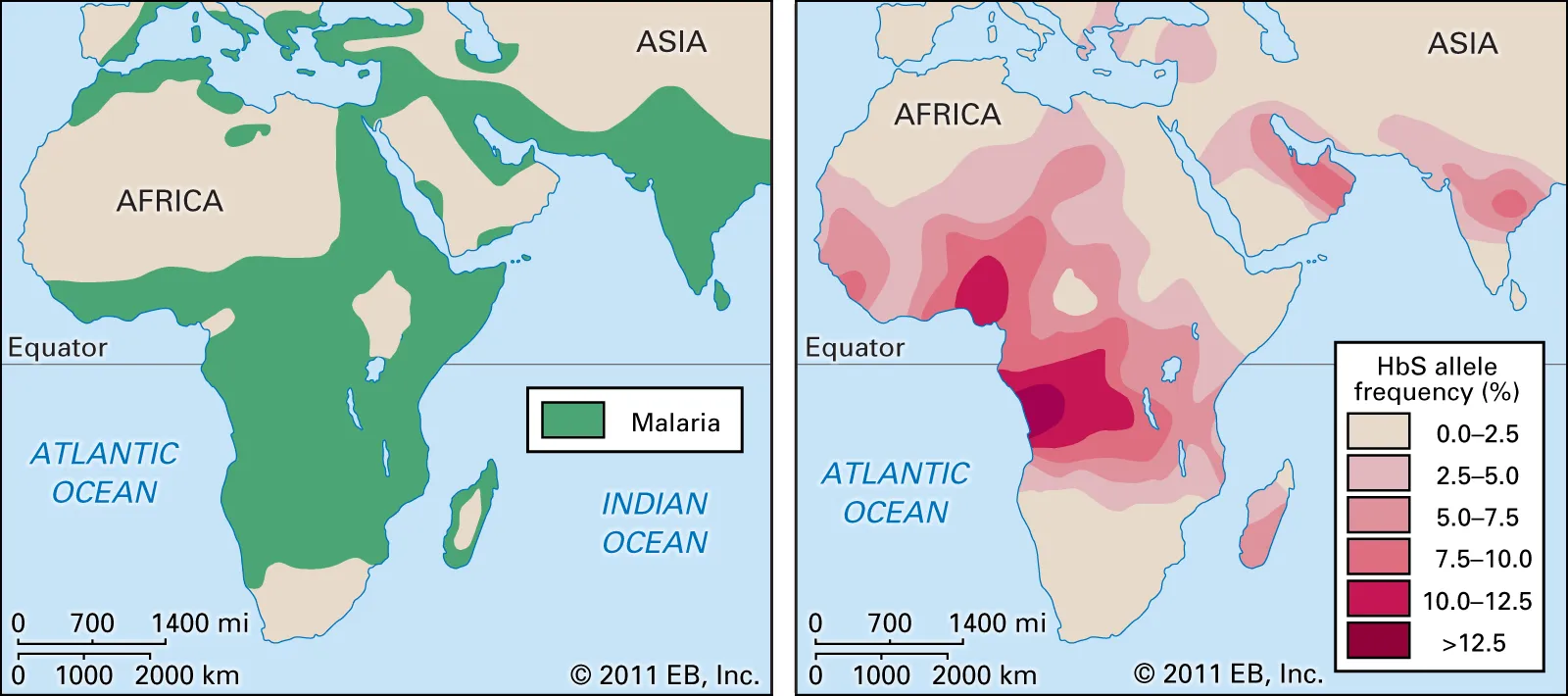
As discussed, SCA does indeed change the structure of blood cells. In the 1950s, a researcher working in Kenya demonstrated that SCA prevalence correlates closely with Malaria prevalence. He also showed that people with sickle cell trait seemed to contract malaria less frequently. Subsequent research has found that trait carriers can and do contract malaria but rarely the most severe form. In fact, they are 90% less likely than non trait carriers to get severe SCA (Kato et al 2018:2). If they do get SCA, they rarely die from it, even from the more severe kinds. It seems that red blood cells in sickle cell trait carriers do get sick, but they are then removed by white blood cells (Luzzatto, 2012).
So it looks like sickle cell anaemia is the product of an aeons-old battle between the malaria parasite and humans. In order to counter the threat of malaria, our people developed a genetic variant which protected us from the effects of malaria if you carried the trait (i.e. one mutated gene). However, if you inherit two mutated genes, while you are presumably still protected from malaria, you now face the debilitating effects of SCA.
References:
Kato G. et al. (2018). Sickle Cell. Nature Reviews Disease Primers volume 4(18010). [Link to abstract: https://www.nature.com/articles/nrdp201810 accessed 2 Sept. 18]
Luzzatto. L. (2012). Sickle Cell Anaemia and Malaria. Mediterranean Journal of Hematology and Infectious Diseases, 4(1). [Link to full article: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3499995/ accessed 2 Sept. 18]
Piel, F. et al. (2017). Sickle Cell Disease. The New England Journal of Medicine, 376(16). [Link to abstract: https://www.nejm.org/doi/full/10.1056/NEJMra1510865 accessed 2 Sept. 18]
